Temperature monitoring for the pharmaceutical industry
Temperature monitoring for the pharmaceutical industry
Medicines, vaccines, samples, and raw materials must be stored between +2 °C and +8 °C (or according to their specifications) during both storage and transport.
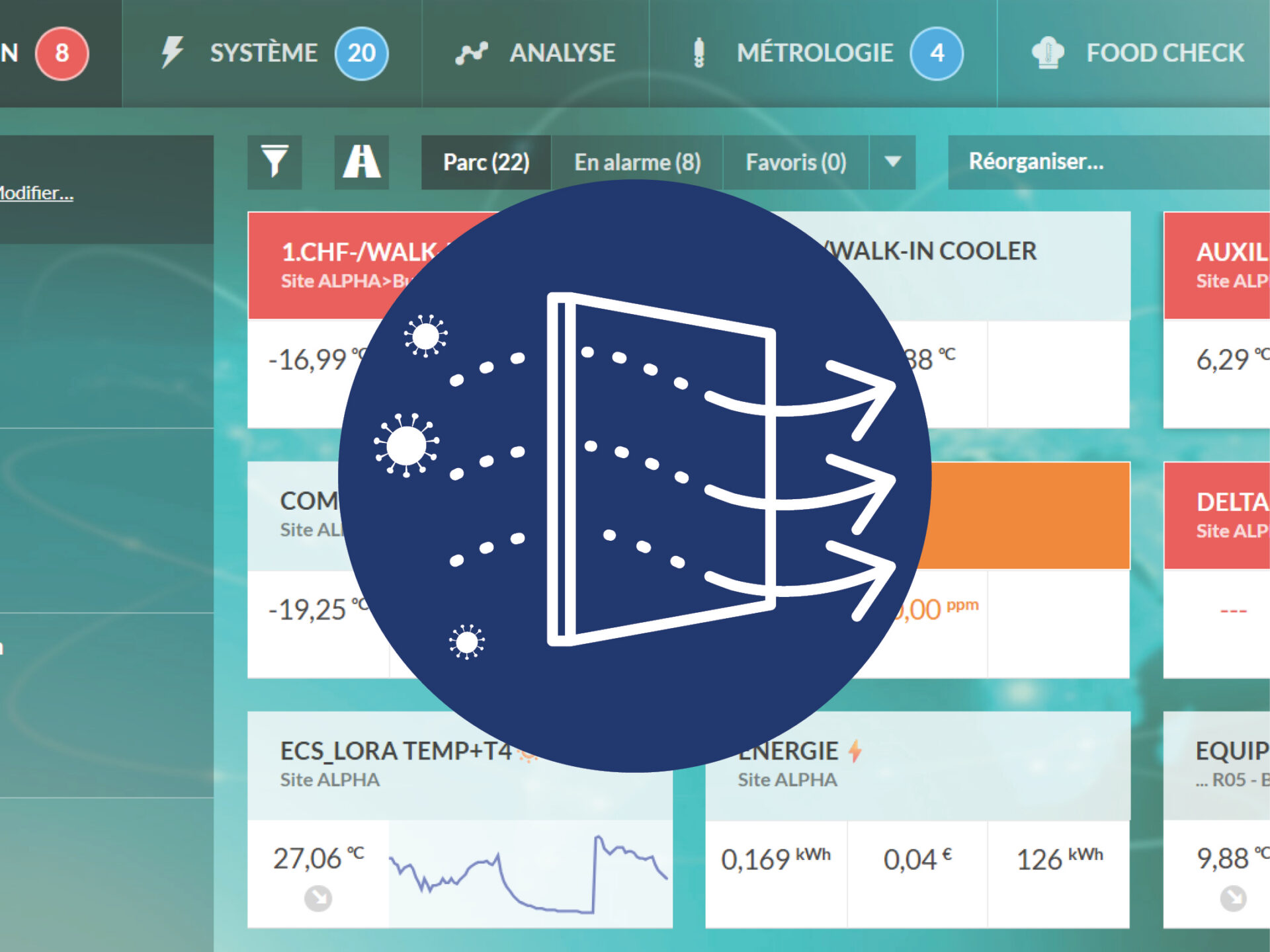
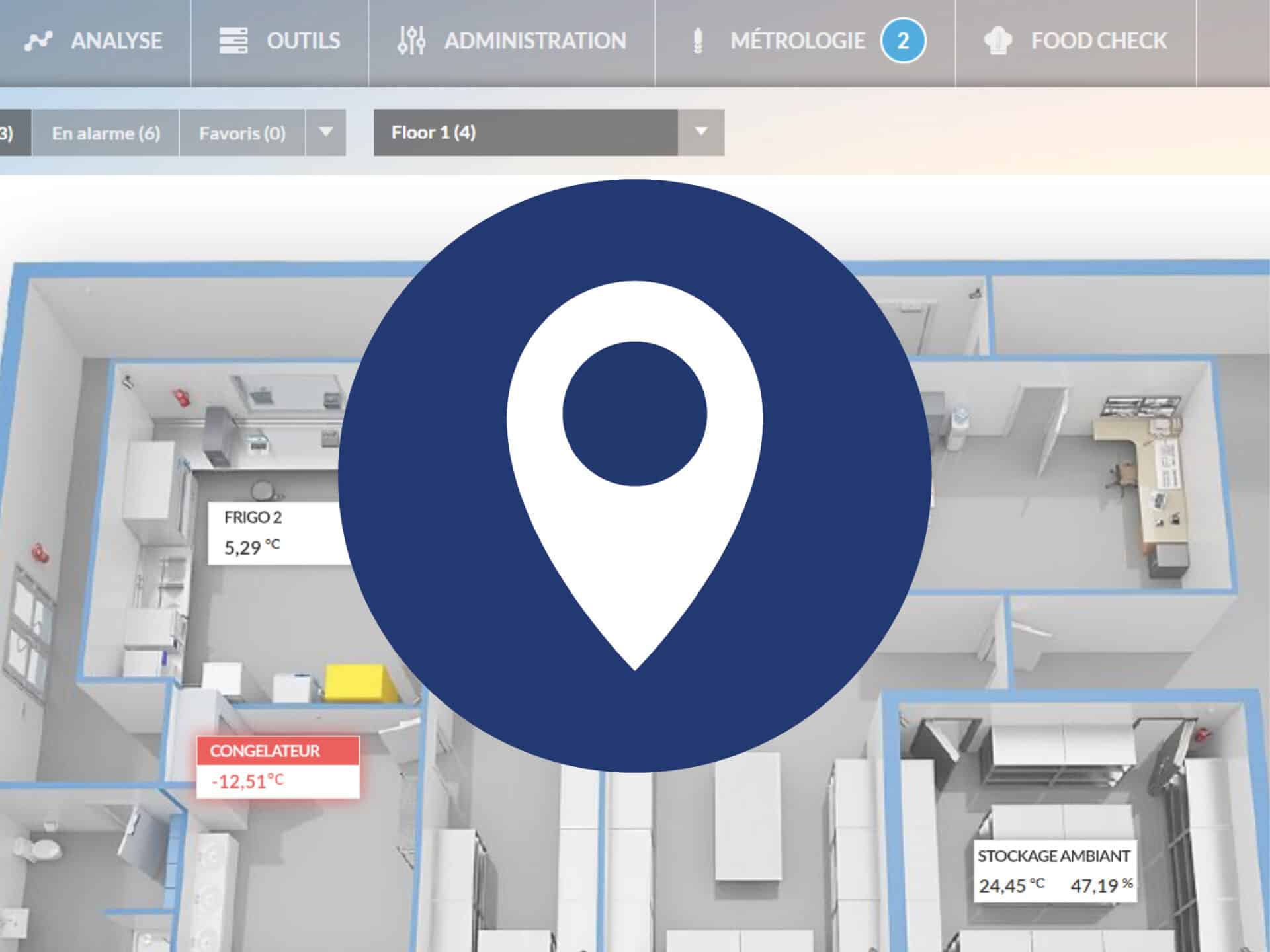
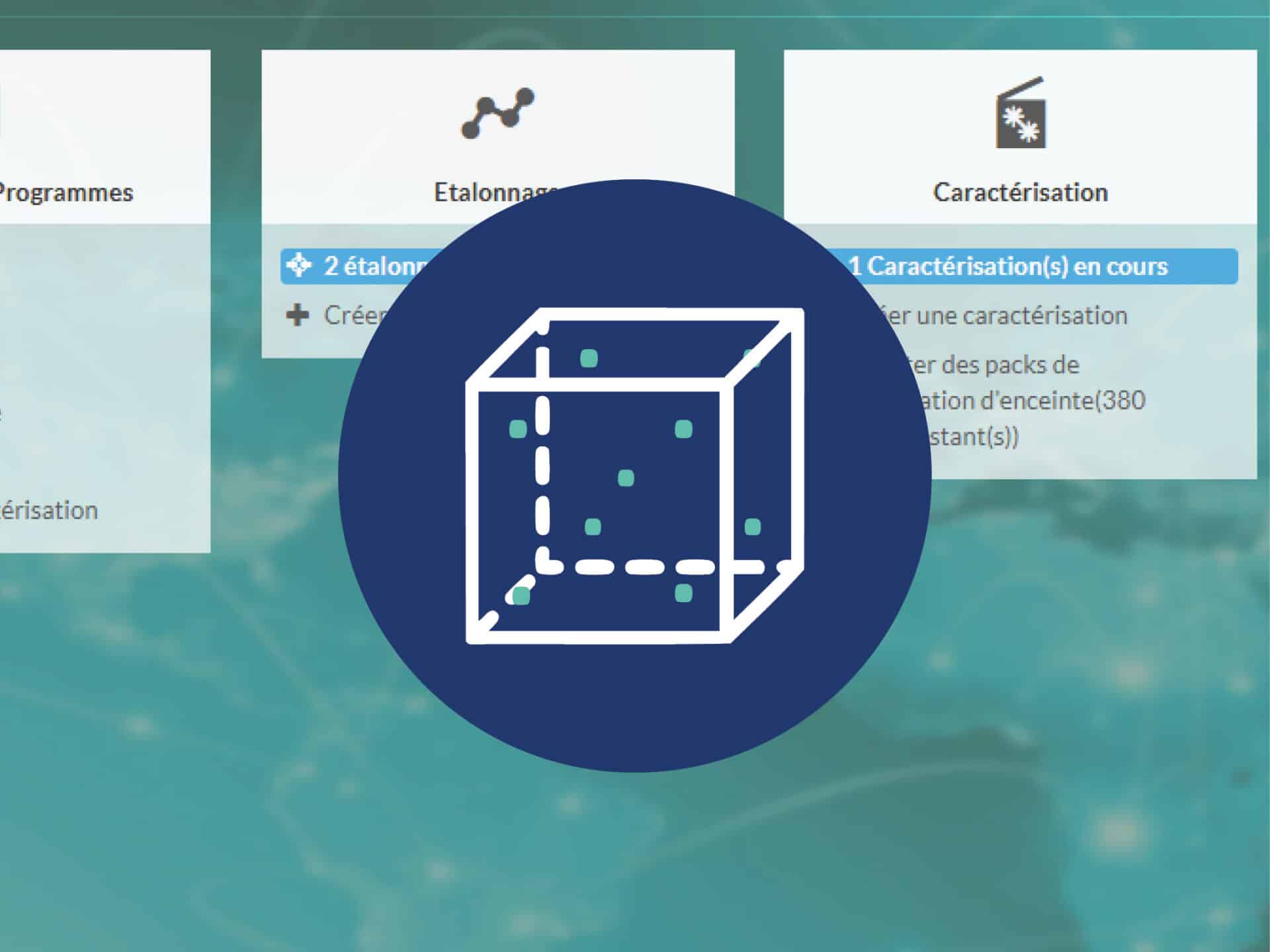
JRI designs IoT solutions for temperature/humidity monitoring: wireless sensors, data loggers, real-time alerts, and secure cloud platforms. The systems comply with ISO 17025, ISO 15189, and Good Manufacturing and Distribution Practices (GxP).